Country specific information – FINLAND
Printable version of this document:
![]() Vnr Country Specific Information of Finland
Vnr Country Specific Information of Finland
| 16.2.2023 | ||
Country specific information – FINLAND
Content:
- Notifications for the Finnish ePrescription database
- National article numbers in Finland
- Pricing of the Finnish Vnrs
- Product codes in Finland
- Contact information
- Notifications for the Finnish ePrescription database
The ePrescription database includes all the necessary and current information that is needed in prescribing and delivering medicines. The ePrescription database contains pharmaceuticals (preparations with a Vnr number) and preparations without a Vnr number (preparations with a temporary special sales license and reimbursed emollients and reimbursed clinical nutrient preparations).
The content of the ePrescription database is covered in section 22 of the Act on Electronic Prescriptions (laki sähköisestä lääkemääräyksestä 61/2007). Social Insurance Institution of Finland (Kela) maintains the ePrescriprion database. Finnish Medicines Agency (Fimea) delivers the information needed for identifying the preparations.
The content of the ePrescription database and the communication to the Kela is covered by section 8 of the Decree of the Ministry of Social Affairs and Health (485/2008).
The Pharmaceutical Information Centre provides the ePrescription database for Kela.
The holder of the marketing authorization, the manufacturer or the importer must communicate the wholesale prices of
- pharmaceuticals
- preparations with a temporary special sales license
- reimbursed emollients
- reimbursed clinical nutrients
to the Pharmaceutical Information Centre, which, under commission from Kela, serves as the technical administrator of the database in respect to pharmaceuticals.
The information must be communicated no later than 8 working days before the 1st and 15th of the month in question by 4 pm Finnish time. The more detailed communication schedule and instructions can be found in Vnr extranet service (https://vnr.fi).
All the notification forms can be filled in the Vnr extranet, Notification forms. (https://vnr.fi).
From there you can choose the notification form needed on the left side of the page.
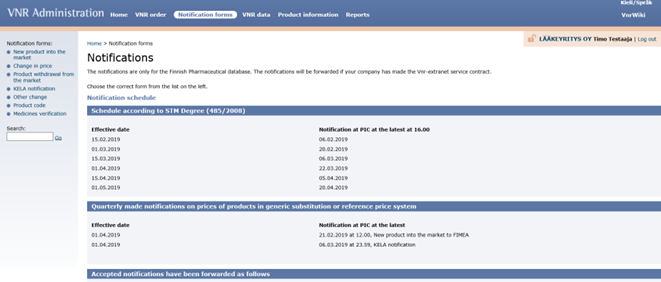
The communicating party is responsible for the accuracy of the price information communicated.
In addition to the Vnr extranet service the “New product into the market” and “Product withdrawal from the market” -notifications must be communicated to the Fimea and the “Change in price” -notifications to the wholesaler in question, The Association of Finnish Pharmacies and to the University Pharmacy. Kela notifications must be made quarterly of the prices of substitutable pharmaceuticals and of pharmaceuticals covered by the reference price system.
Due to the upcoming Falsified Medicines Directive, a new medicines verification notification was introduced in the Vnr Service 18.6.2018. Pharmaceutical companies make this notification like other notifications. Pharmaceutical Information Centre delivers the information of the notifications to wholesalers and pharmacy/hospital pharmacy system suppliers to ensure efficient and reliable action.
Companies with a Vnr-extranet service contract
The Pharmaceutical Information Centre will deliver the information in question to the University Pharmacy, the Association of Finnish Pharmacies, sales statistics (Finnish Pharmaceutical Data and IMS Health Finland), wholesalers (Oriola, Tamro, Magnum medical), the Social Insurance Institution of Finland, the Pharmaceutical pricing board and FIMEA on behalf of companies that have a Vnr-extranet service contract. Pharmaceutical Information Centre is responsible for the delivery of data to only those third parties mentioned above which are entitled to this information before it becomes public.
- National article numbers in Finland
The Nordic article numbers, Vnrs, are only allocated for registered pharmaceuticals.
Please note that sample medicines and radiopharmaceuticals don’t need a Nordic article number in Finland.
All other products, such as reimbursed products with special permission for compassionate use, products with temporary special permission for compassionate use, medical devices and consumer products that need an article number, use national numbers. The national numbers are given by the wholesalers that distribute the products.
The three largest wholesalers in Finland are Tamro (www.tamro.fi), Oriola (www.oriola.fi) and Magnum Medical (www.magnummedical.fi).
- Pricing of the Finnish Vnrs
Nordic article numbers, allocated for Finland, are invoiced by the Pharmaceutical Information Centre, PIC.
New Vnrs are invoiced with an opening fee after assignment. Please note that the opening fee will be invoiced even if the Vnr should be withdrawn shortly after the assignment.
All active Vnrs are also invoiced annually. The annual fee is invoiced in the beginning of the calendar year.
In Finland the Vnr fees include, except the administration of the numbers, also the Vnr extranet service with the notification forms for the Finnish ePrescription database and the possibility to make a contract with PIC for forwarding the information to authorities and other parties.
We highly recommend the companies to regularly check their active Vnrs and withdraw all unnecessary numbers.
The companies are also obliged to inform us about any changes in contact information regarding the invoicing. Invoices that have already been sent out are not credited.
Please contact vnr@vnr.fi for more information regarding current fees and invoicing.
- Product codes in Finland
The information on the linkage between Vnr and up to date product codes (GTINs, unique NTINs) is essential for the correct handling of the packs at the pharmacies, hospital pharmacies and wholesalers with or without FMD verification. In the Vnr Service you can maintain information about both outer pack product codes and immediate (inner) pack product codes.
Product code notification
You can easily inform about a new and changed product codes with the product code functionality. New product codes can be added with product code notification in the notification section (Notification forms > Product code). Product codes can be added for a single product or, alternatively, a file containing many product codes for many Vnrs can be uploaded. The file upload option is available for outer pack product codes only.
– Compulsory information in the notification: Country (FI, SE, NO, DK, IS), Vnr and product code
– Optional field: Comment (useful for example for hospital pharmacies, wholesalers etc. For immediate pack codes it is possible to specify which immediate pack includes the code, for example the solution or the powder). Can also be left empty.
– A different product code can be added to each country where the Vnr is used
– One Vnr can have more than one product code in each country at the same time
N.B! You are able to see also other notifications in the notifications section.
Very important: All other notification forms (=New product into the market, Change in price, Product withdrawal from the market, KELA notification and Other change) concern ONLY FINNISH market.
Product code reports to manage your product code data
You can get a report of all product codes of the products of your company. You can find the new Product code report in the report section of Vnr Extranet Service (Reports > Product codes). The report includes all product codes informed to the Vnrs as well as the country and additional information of the product codes.
For outer pack product code reporting needs of a single Vnr you can also use the current normal pdf-receipt. The Vnr receipt has been updated so that the most recently added outer pack product code for each country is shown.
PIC forwards the product code information to co-operation parties
When you do a product code notification, we forward the new and updated outer pack product code information to all necessary co-operation parties (pharmacy registers, wholesalers and statistic companies) as other notifications, if you have Vnr Extranet Service agreement in force. Product code information is also included in the “PF Lääkerekisteri” (used by e.g., hospital pharmacies).
Both outer and immediate pack product codes are forwarded to the major Finnish hospitals who use Pharmaceutical Information Centre’s databases in their systems. This enables closed medication loop in hospitals.
Fimea advice that if the package is provided with a product code or other corresponding identifier, it should be printed so that the label added by the pharmacy won’t cover it.
- Contact information
Pharmaca Health Intelligence Oy
(Lääketietokeskus Oy)
Korkeavuorenkatu 35
FI-00130 Helsinki
Finland
Tel. +358 9 6150 4950
http://pharmaca.fi/en
vnr@vnr.fi
Fimea
The Finnish Medicines Agency Fimea is the national competent authority for regulating pharmaceuticals
Postal address: P.O. Box 55, FI-00034 FIMEA, FINLAND
Delivery address: Nauvontie 4, 00280 Helsinki, Finland
Microkatu 1, 70210 Kuopio
Itsenäisyydenaukio 2, 20800 Turku
Switchboard: +358 29 522 3341
E-mail: laakerekisteri@fimea.fi
Web site: http://www.fimea.fi
HILA
The Pharmaceuticals Pricing Board is subordinated to the Ministry of Social Affairs and Health, Insurance Department.
The Pharmaceuticals Pricing Board confirms reimbursement and a reasonable wholesale price of
- medicinal products
- clinical nutritional preparations
- basic ointments
that are reimbursable under the Health Insurance Act.
The Pharmaceuticals Pricing Board also monitors the effects of reimbursement and wholesale price decisions on reimbursement costs.
Mailing address: P.O. Box 33, FI-00023, Government, Finland
Visiting address: Snellmaninkatu 13, 5th floor, Helsinki
Phone: +358 295 16001 (switchboard; Government)
Fax: +358 9 698 1191
Email: hila@stm.fi
Web site: www.hila.fi
KELA
Kela operates under the supervision of Parliament.
Kela is responsible for providing the National Archive Health Information (KanTa) services. KanTa is a collective term used for a range of national health care information systems including ePrescription, eArchive, a national pharmaceutical database, and a portal for citizens to access their own health information online.
Mailing address: PL 78, 00381 Helsinki
Visiting address: Höyläämötie 1 a B, 00380 Helsinki
Phone: +358 20 634 11
http://www.kela.fi/generic-substitution-and-the-reference-price-system-timetable-for-price-notifications
http://www.kela.fi/pharmaceuticals-database
SAL (AFP)
Maintains and produces information about medicinal products and their prices for pharmacies
Suomen Apteekkariliitto/ Association of Finnish Pharmacies
Pieni Roobertinkatu 14
FI-00120 Helsinki, Finland
Tel. +358 10 6801400
Fax. +358 9 647 167
E-mail: taksa@apteekkariliitto.fi
Web site: http://www.apteekkariliitto.fi
YA
Maintains and produces information about medicinal products and their prices for pharmacies
Yliopiston Apteekki/ University Pharmacy
Valimotie 7
P.O. Box 17
00381 Helsinki
Phone: +358 9 542 046
Fax: +358 9 5420 4456
E-mail: tuoterekisteri@yliopistonapteekki.fi
Web site: http://www.yliopistonapteekki.fi
