Country specific information – SWEDEN
Printable version of this document:
![]() Vnr Country specific information of Sweden
Vnr Country specific information of Sweden
LIFs recommendation – Transition from NTIN to GTIN:
![]() English /
English / ![]() Swedish
Swedish
In this document information related to the handling of article numbers in Sweden is presented. Definitions of keywords are found in chapter 10.
Nordic & National article numbers
Country specific information
Sweden
Version 1.5
Purpose of the document
This document contains the Swedish country specific information presented in VnrWiki and will be used when there is a need to update the Swedish country specific information in VnrWiki.
A new version of the document with updated information visualised with track changes should be sent to PIC, who will implement the changes in VnrWiki.
Thereafter, the track changes are accepted, and the document saved to be used for the next update of the information in VnrWiki.
Index
Article numbers – Country specific information – Sweden_ 5
1 The flow of product and article information_ 5
2 Vnr – stakeholders’ different needs_ 8
2.1 Medical Product Agency – Läkemedelsverket (regulatory control)_ 8
2.3 The Swedish eHealth Agency (VARA – a national product and article register)_ 9
2.4 Wholesale distributors (distribute)_ 9
2.5 Health care (prescribe)_ 10
2.6 Pharmacies (logistics and dispensing)_ 10
3 Impact on Vnr when an article and/or its package changes_ 11
3.1 What happens when an incorrect Vnr has been saved in LiiV?_ 11
3.3 Change of marketing authorisation holder (MAH) 13
3.4 Change of package type_ 13
3.6 Can old Vnrs be removed from LiiV for articles, which are not marketed?_ 14
3.8 How is the previous Vnr handled in LiiV when a Vnr is changed?_ 14
5 Vnr – layout and allocation_ 19
6 Pricing of Nordic Article Numbers_ 19
7 Use of article number series_ 20
8 Läkemedelsbranschens nummernämnd, LNN_ 22
Article numbers – Country specific information – Sweden
1 The flow of product and article information
The Swedish eHealth Agency has the national responsibility to compile and supply a national product and article register to the Swedish market. The purpose of the register, called VARA, is to supply the Swedish market with up to date and quality assured product and article information for the approved pharmaceuticals on the market in Sweden and consumer products within the reimbursement system.
VARA is distributed to all pharmacies, veterinarian health care systems, system suppliers, statistics stakeholders and Sil, Svenska Informationstjänster för Läkemedel. Sil in turn distributes the VARA information to health care actors. All health care systems should be integrated with SIL.
Figure 1 shows the VARA system at the Swedish eHealth Agency and its sources. VARA daily collects and compiles updates from LiiV.
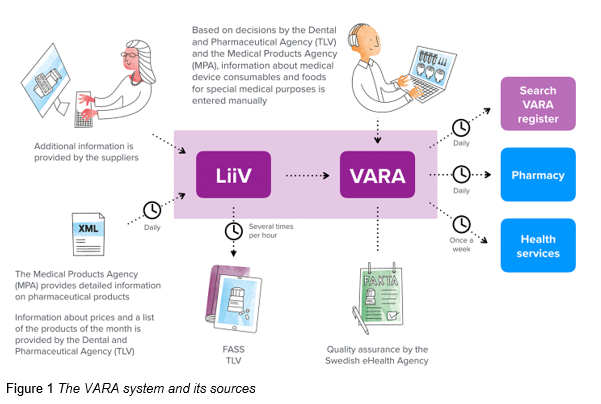
The quality of data in VARA is vital, and the information in VARA is therefore updated in accordance with a well-defined input process. All changes are validated with automatic validity checks and are thereafter reviewed and approved by pharmacists before they are published once a day.
It is the responsibility of the information owners, such as TLV, MPA (LV) and pharma companies to deliver quality assured information to VARA and quickly correct any deviations.
Pharmacies are required to update their systems with information from VARA daily in order to be able to file prescriptions from National Medication List, NLL at the Swedish eHealth Agency.
Currently Sil and health care systems are updated with VARA information approximately once a week.
Figure 2 shows how dispensing systems at pharmacies use a local copy of VARA and how central systems such as the Prescription repository (National Medication List, NLL/receptdepå djur, RDD) and the Register of sales transactions (Försäljningstransaktionsregistret, FOTA) at the Swedish eHealth Agency are directly linked to VARA. The figure schematically also shows how health care actors have access to VARA via Sil alternatively for veterinarian health care systems directly from the Swedish eHealth Agency, and how electronic prescriptions get into RR/RRD.
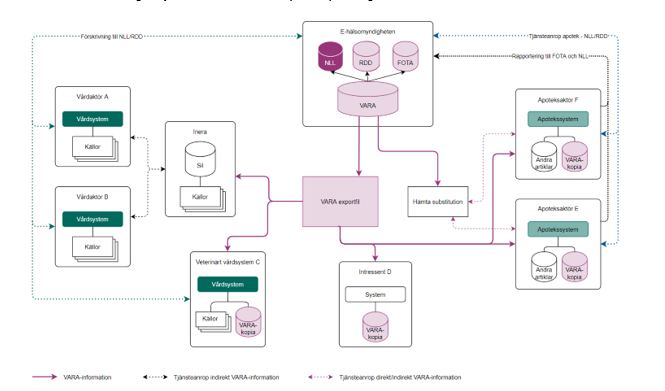
| Swedish | English |
| Sil Sil-databas Andra källor |
Sil Sil database Other sources |
| Vårdaktör A-B Vårdsystem |
Health care actor A-B Health care system |
| Veterinärt vårdsystem C Vårdsystem |
Veterinarian health care actor C Health care system |
| Apoteksaktör E-F Expedierande system Andra artiklar VARA-kopia |
Pharmacy Actor E-F Dispensing system Other articles VARA (copy) |
| Intressent D Övrig intressent |
Stakeholder D Other stakeholder |
| Förskrivning till NLL/RDD | Prescription to NLL/RDD |
| Tjänsteanrop apotek – NLL/RDD | Service calls pharmacy – NLL/RDD |
| Rapportering till FOTA och NLL | Reporting to FOTA and NLL |
Figure 2 VARA, pharmacies, and health care actors
2 Vnr – stakeholders’ different needs
This chapter describes how different stakeholders use, and are dependent on, Vnrs in the handling of pharmaceuticals and reimbursed consumer products on the Swedish market.
In Sweden there is not a single identification code, which provides a unique identity for all pharmaceuticals, medical devices, and consumer products. However, the Vnr has, in combination with other identification codes, a very important function in the handling of pharmaceuticals on the Swedish market.
It is extremely important that manufacturers working with products on the Swedish market are aware that the Vnr is a critical attribute. It functions as an information carrier between a number of IT-systems involved in the prescription, dispensing, ordering, storage and information regarding medicines. The usage of the Vnr is therefore a matter of well-functioning management of articles as well as patient safety. In addition, electronic prescriptions as well as highly automated management systems in all parts of the distribution chain enhance the risk of fatal consequences if a Vnr is mixed or incorrect.
For the distribution chain to function safely, it is important to know when and how an article should change Vnrs.
An error in a Vnr may result in unexpected and serious consequences. The result of an incorrect Vnr can be that a pharmaceutical loses its identification or is identified as a completely different pharmaceutical. After the reregulation of the pharmacy market the systems of the stakeholders have become highly automated and integrated, which further increases the need for correct Vnrs for the management of pharmaceuticals to function efficiently and safely.
In Sweden, the handling of Vnrs is funded through the FASS-charge and therefore there is no direct cost associated with acquiring a Vnr.
The administration of the Vnr system is under responsibility of the Nordic Number Centre, (NNC)[1], vnr@vnr.fi, located at Pharmaceutical Information centre Ltd. (Lääketietokeskus Oy), Helsinki, Finland.
2.1 Medical Product Agency – Läkemedelsverket (regulatory control)
The Medical Product Agency (Läkemedelsverket) use NPL id and NPL pack id as identification codes. Vnrs are not used by the MPA, but in LiiV there is a field for Vnr, which the responsible company adds together with other information.
2.2 TLV (prices)
Please read TLV’s document Handbok för företag vid ansökan om subvention och pris för läkemedel. The document is available on the website of TLV: Handbok för företag vid ansökan om subvention och pris för läkemedel (tlv.se)
2.3 The Swedish eHealth Agency (VARA – a national product and article register)
The Swedish eHealth Agency has the national responsibility to compile and provide a national product and article register to the Swedish market. The purpose of the register, called VARA, is to provide the Swedish market with up to date and quality assured product and article information for the approved pharmaceuticals on the market in Sweden and consumer products within the reimbursement system.
It is the responsibility of the information owners, such as TLV, MPA (LV) and pharma companies to deliver quality assured information to VARA and quickly correct any deviations.
Since information in LiiV is automatically distributed via VARA to the various stakeholders in the market, which in turn uses the information in a variety of situations and applications, it is very important that the Vnrs saved in LiiV are correct. Changes in LiiV are transferred and updated automatically in subsequent systems. Please be very careful when entering the Vnr. Notify the Swedish eHealth Agency and correct immediately if an error occurs. When the flag “On the market” once has been set and saved to “yes” the article information will always be distributed from VARA.
If an incorrect Vnr has been saved in LiiV, and it is discovered later, prescription on the Vnr may have occurred. In such a situation – always contact the Swedish eHealth Agency to discuss how the situation should be handled. It is very important not to delete, edit or otherwise modify the incorrect information without first consulting with the Swedish eHealth Agency, since it can have major consequences in subsequent systems.
When a pharma company is about to change a specific Vnr, it is important that the change of Vnr will be synchronised with the distributor. The reason for this is to achieve time to synchronize the change of Vnr between the pharmacies and the distributor in order to maintain the whole distribution chain. If the change of Vnr is done too early or too late in LiiV, there is a risk that the pharmacies purchase orders won’t be recognised by the distributor.
2.4 Wholesale distributors (distribute)
Currently the distribution systems of the two main distributors in the Swedish market, Oriola and Tamro, use Vnrs to identify articles.
Vnrs are used throughout the whole distribution chain from pharma companies to the patient, e.g. purchase orders, inventory management and traceability according to GDP. In addition, all pharmacies currently order on Vnrs.
When the same Vnr is used in several Nordic countries for an identical article where only the product information leaflet in the packages is different there are problems in the handling of the article, since it is not visible on the package for which country the package is intended. If an article is sent to the wrong country, it results in withdrawals. This problem is handled with different GTINs for the different markets.
Please see more information about GTIN/NTINs.
2.5 Health care (prescribe)
Currently the handling of pharmaceuticals in health care would not function without Vnrs.
Most health care systems currently use Vnrs to identify pharmaceuticals when medicines are prescribed. It is therefore important that the Vnrs are updated and correct.
To be able to follow the information about a pharmaceutical over time, it is important that the article history of Vnrs is complete and clear, and that as few changes as possible are made to the article.
Please see more information about GTIN/NTINs.
2.6 Pharmacies (logistics and dispensing)
Vnrs are necessary for several of the processes in pharmacies. They play a central role in
- Ordering from different pharmaceutical distributors and all parts of stock management in all kinds of pharmacies
- Dispensing with the support of the IT-systems for prescription dispensing in retail pharmacies, dose dispensing pharmacies, hospital pharmacies and distance pharmacies
- Ordering and dispensing of e-prescriptions, customer service etc.
- Management of article information in article repositories and article information in intra- as well as internet information systems
- Ordering systems for hospitals
Due to the important role of Vnrs in the management of prescribed drugs it is crucial that the Vnr is correct, showing the article it belongs to and being transferred in a safe and correct way between systems.
3 Impact on Vnr when an article and/or its package changes
For the common Nordic guidelines on changes, see Nordic instructions. Listed below is additional country specific information for Sweden on how a change in an article and/or its package should be handled. Any questions can be directed to the Swedish eHealth Agency servicedesk@ehalsomyndigheten.se
NOTE – All changes of an article and its package must be informed to, and confirmed with, NNC/PIC in advance.
The general rule is that the Vnr must be changed if any of the six basic criteria has been changed, or no longer is valid, see Nordic instructions.
In many cases it is not mandatory, but most often recommended, to change the Vnr when other criteria than the six basic changes. By changing Vnr it will be obvious to all stakeholders that a change has been made to the article. Please see more information about GTIN/NTINs.
Changes can easily be made for Vnrs, which never have been on the market (status Assigned) in any country. Only in very special cases and rarely can a Vnr be kept when an article or its package, which has been on a market, is changed. Usually, a new Vnr is required.
Pharmaceutical companies have a responsibility to always inform about changes in trade name, marketing authorisation holder, and Vnr to all pharmacies, see Contact information.
For NNC to make correct decisions it is important that the status of an article in each country is correct in the Vnr system. It is the responsibility of the company to inform NNC about all changes.
Please read TLV’s document Handbok för företag vid ansökan om subvention och pris för läkemedel. The document is available on the website of TLV: Handbok för företag vid ansökan om subvention och pris för läkemedel (tlv.se)
3.1 What happens when an incorrect Vnr has been saved in LiiV?
Since information in LiiV is automatically distributed via VARA to the various stakeholders in the market, which in turn uses the information in a variety of situations and applications, it is very important that the Vnrs saved in LiiV are correct. Changes in LiiV are transferred and updated automatically in subsequent systems. Please be very careful when entering the Vnr. Notify the Swedish eHealth Agency immediately if an error occurs and make the correction in consultation with them. When the flag “On the market” once has been set and saved to “yes” the article information will always be distributed from VARA.
It takes a business day until a change in LiiV is available in the dispensing systems. In health care systems it takes approximately 1-2 weeks before the updated information becomes available to prescribers. Information in FASS.se is updated from LiiV several times a day. See Chapter 1, The flow of product and article information.
If an incorrect Vnr has been saved in LiiV, and it is discovered later, prescription on the Vnr may have occurred. In such a situation – always contact the Swedish eHealth Agency to discuss how the information should be handled. It is very important not to delete, edit or otherwise modify the incorrect information without first consulting with the Swedish eHealth Agency, since it can have major consequences in subsequent systems.
When a pharma company is about to change a specific Vnr, it is important that the change of Vnr will be synchronised with the distributor. The reason for this is to achieve time to synchronise the change of Vnr between the pharmacies and the distributor to maintain the whole distribution chain. If the change of Vnr is done too early or too late in LiiV, there is a risk that the pharmacies purchase orders won’t be recognised by the distributor.
3.2 Change of trade name
It is recommended that the Vnr is changed when the trade name of the product changes. It will thereby be obvious to all stakeholders that the article somehow has been changed.
If agreed upon in advance with NNC the Vnr, in certain situations, can be maintained:
- The article only exists in one country
- The trade name change takes place more or less at the same time (within a few months) in all Nordic countries. If it takes longer the Vnr must be changed.
In cases when a Vnr is to be changed, packs with different trade names but with the same Vnr can be marketed in Sweden during a transition period (one month), when agreed with the MPA (LV).
If a company wants a longer period of parallel sale than a month it must be discussed and approved by the MPA contact person who administers the name change. A longer period is in principle never accepted unless it is a very small change, for instance that the suffix of “vet” is added to, or removed from, a vet. trade name.
For pharmaceuticals with generic names longer parallel sales can, in certain cases, be accepted.
The pharmaceutical company has a responsibility to inform all pharmacies about the change of a Vnr.
3.3 Change of marketing authorisation holder (MAH)
The same Vnr can be maintained if the MAH is changed at the same time in all Nordic countries and if the old and the new MAH agree on this. It should be noticed that the sales statistical history moves to the new MAH together with the Vnr.
When a product changes MAH all other but withdrawn Vnrs associated with the product must be moved to the new MAH. Thereafter the Vnrs can be continuously used or changed. The status of not needed Vnrs may be changed to Withdrawn, but the Vnrs may not be deleted from LiiV.
When a Vnr is withdrawn there is no longer a cost associated with the Vnr. In Sweden, the handling of Vnrs is funded through the FASS-charge and therefore there is no direct cost associated with Vnrs.
Please contact NNC to discuss the specific situation.
The pharmaceutical company has a responsibility to inform about the change of a Vnr to all pharmacies.
3.4 Change of package type
In most cases the Vnr needs to be changed. Please contact the Swedish eHealth Agency and/or NNC when considering changing package type or package material. A new package gets a new NPL pack id.
A change of NPL pack id affects the price in the reimbursement system. If the old package was reimbursed, the company needs to send a new application for reimbursement for the new package to TLV. Information regarding the application procedure can be found in TLV’s document Handbok för företag vid ansökan om subvention och pris för läkemedel. The document is available on the website of TLV: Handbok för företag vid ansökan om subvention och pris för läkemedel (tlv.se)
If a company, without applying for price within the reimbursement system and getting the price approved by TLV, moves a Vnr in LiiV to a new NPL pack id, the price within the reimbursement system will not automatically be updated. This means that the package will be handled as if it is not reimbursed in the systems of the pharmacies.
3.5 Change of Rx / OTC
An OTC-package is by definition a different package compared to the corresponding Rx-package and must therefore carry different Vnrs if both packages are on the market at the same time.
If a switch is made from RX to OTC the Vnr should be changed, since the requirements for package text, package leaflet etc. differ.If a switch is made from OTC to Rx the Vnr can be maintained.
3.6 Can old Vnrs be removed from LiiV for articles, which are not marketed?
Once an article has been set as “On the market = Yes” in LiiV the specific Vnr of that article may not be removed / blanked out in LiiV. Sales transactions are linked to the Vnrs in many systems, such as the National Medication List (NLL) and statistical systems within and outside the Swedish eHealth Agency. To mark a change for an article that is not sold, for instance when an article has been passed to another MAH, it is permissible to acquire a new Vnr, see Application for Nordic Article Number.
In Sweden, the handling of Vnr is funded through the FASS-charge and there is therefore no economic reason to refrain from acquiring a new Vnr.
3.7 May a Vnr be reused?
It is absolutely forbidden to reuse a Vnr for another article. An article, which has been prescribed and dispensed, is registered with the Vnr in several systems, e.g. the National Medication List, statistics, distribution systems, order and supply systems, article history and invoicing.
For correct historical handling the Vnr must remain even though the article is not marketed.
If a company reuses a Vnr and already has printed packages, these packages may not under any circumstances be distributed on the Swedish market! The company must acquire a new Vnr, and thereafter print new packages.
In some cases, NNC may reactivate a withdrawn Vnr if the article is exactly the same and if all the information is still relevant. This can only take place after direct contact with, and decision by, NNC.
3.8 How is the previous Vnr handled in LiiV when a Vnr is changed?
When changing a Vnr in LiiV, the replaced Vnr will automatically be moved to the field “Previous item number”.
The previous Vnr will be saved in VARA together with the new one. This makes it possible for the pharmacies to sell both the old and the new package simultaneously.
3.9 May an identical parallel imported article originating from different export countries have the same Vnr in a country and in different countries?
Identical parallel imported pharmaceuticals originating from different export countries should have the same Nordic Article Number in Sweden.
If they fulfil the conditions for common Nordic packages, they could have the same Vnr in the different Nordic countries despite differing MT-numbers.
4 Checklists
4.1 New Vnr
| # | Activity | Comment | To do | Reference |
| 1 | New Vnr | Application for a Nordic Article Number should be made when the MAH knows which packages will be marketed and really need Vnrs.
Technically Vnrs can be applied for at any time. However, a Vnr should be used on the market within three years. Otherwise, the status of the Vnr will be changed to Withdrawn. Six basic criteria are mandatory and need to be met equally in all countries concerned if a Vnr is to be used in more than one country. |
Order Vnr: https://vnr.fi | Application for Vnr |
4.2 Change of Vnr
| # | Activity | Comment | Todo | Reference |
| 1 | Change Vnr? | Changes can easily be made for articles and Vnrs, which never have been on the market (status Assigned) in any country. Only in very special cases and rarely can a Vnr be kept when an article or its package, which has been on a market, is changed. Usually, a new Vnr is required.
All changes of an article and its package must be informed to, and confirmed with, NNC/PIC in advance. Six basic criteria are mandatory and need to be met equally in all countries concerned if a Nordic Article Number is to be used in more than one country. |
Contact the Swedish eHealth Agency and/or NNC/PIC to discuss if the Vnr should/ought to be changed when an article or its package is changed.
Order Vnr: https://vnr.fi Thereafter, if agreed, the Vnr is updated in LiiV. When changing a Vnr in LiiV, the replaced Vnr will automatically be moved to the field “Previous item number”. |
Nordic instructions
Swedish guidelines – chapter 3 TLV information: Handbok för företag vid ansökan om subvention och pris för läkemedel. |
| 3 | Change of trade name | It is recommended that the Vnr is changed when the trade name of the product changes.
If agreed upon in advance with NNC the Vnr, in certain situations, can be maintained: · The article only exists in one country · The trade name change takes place more or less at the same time (within a few months) in all Nordic countries. If it takes longer the Vnr must be changed. |
In cases when a Vnr is to be changed, packs with different trade names but with the same Vnr, can be marketed in Sweden during a transition period (one month) according to a routine at the MPA (LV).
See ”Todo” for ”1. Change Vnr?” above. |
Nordic instructions
Swedish guidelines – chapter 3.2 |
| 4 | Change of marketer/MAH | The same Vnr can be maintained if the MAH is changed at the same time in all Nordic countries and if the old and the new MAH agree on this.
It should be noticed that the sales statistical history moves to the new MAH together with the Vnr. When a product changes MAH all other but withdrawn Vnrs associated with the product must be moved to the new MAH. Thereafter the Vnrs can be continuously used or changed. The status of not needed Vnrs may be changed to Withdrawn, but the Vnr may not be deleted from LiiV. |
Please contact NNC to discuss the specific situation.
See ”Todo” for ”1. Change Vnr?” above. |
Nordic instructions Swedish guidelines – chapter 3.3 |
| 5 | Switch Rx/OTC | An OTC-package is by definition a different package compared to the corresponding Rx-package and must therefore carry different Vnrs if both packages are on the market at the same time.
If a switch is made from Rx to OTC the Vnr should be changed, since the requirements for package text, package leaflet etc. differ. If a change is made from OTC to Rx the Vnr can be maintained. |
See ”Todo” for ”1. Change Vnr?” above. | Nordic instructions
Swedish guidelines – chapter 3.5 |
| 6 | Change of package type | In most cases the Vnr needs to be changed. Please contact the Swedish eHealth Agency and/or NNC when considering changing package type or package material. A new package gets a new NPL pack id.
If a company, without applying for price within the reimbursement system and getting the price approved by TLV, moves a Vnr in LiiV to a new NPL pack id, the price within the reimbursement system will not automatically be updated. This means that the package will be handled as if it is not reimbursed in the systems of the pharmacies. It is important that the change of Vnr will be synchronised with the distributor. The reason for this is to synchronize the change of Vnr between the pharmacies and the distributor to maintain the whole distribution chain. If the change of Vnr is done too early or too late in LiiV, there is a risk that the pharmacies purchase orders won’t be recognized by the distributor. |
Please contact the Swedish eHealth Agency and/or NNC when considering changing package type or package material.
A change of NPL pack ID affects the price in the reimbursement system. If the old package was reimbursed, the company needs to send a new application for reimbursement for the new package to TLV. Information regarding the application procedure can be found in TLV’s document Handbok för företag vid ansökan om subvention och pris för läkemedel. The document is available on the website of TLV: Handbok för företag vid ansökan om subvention och pris för läkemedel (tlv.se) In cases when a Vnr is not changed packs with different package type, but with the same Vnr, can be marketed in Sweden during a transition period (one month) according to a routine at the MPA (LV). See ”Todo” for ”1. Change Vnr?” above. |
Nordic instructions
Swedish guidelines – chapter 3.4 |
4.3 Withdrawal of Vnr
| # | Activity | Comment | Todo | Reference |
| 1 | Withdrawal of an article or a product? | A withdrawal of an article/product is a permanent action.
When the withdrawal date has passed the pharmaceutical or the article will disappear automatically from the reimbursement system and is no longer shown in the price- and decision databases of TLV. |
Contact MPA (LV) who registers a date of withdrawal in LiiV, either for the product or an article.
Inform NNC/PIC that the article/product has been withdrawn in Sweden. |
Nordic instructions |
| 2 | Withdrawal of a Vnr | A Vnr withdrawal can be notified at any time by using the withdrawal function in the Vnr extranet service | Notify by using the Vnr extranet service: https://vnr.fi | How to withdraw a Nordic Vnr? (Tutorial) |
| 3 | Reuse of Vnr | It is absolutely forbidden to reuse a Vnr for another article. | A company, which reuses a Vnr and already has printed packages, may not under any circumstances distribute these packages on the Swedish market!
This means that the company must acquire a new Vnr, and thereafter print new packages. |
Swedish guidelines – chapter 3.7 |
5 Vnr – layout and allocation
In Sweden, the Vnr is required on the outer labelling of all medicinal products, except radiopharmaceuticals and homeopathic medicinal products.
5.1 Layout of the Vnr
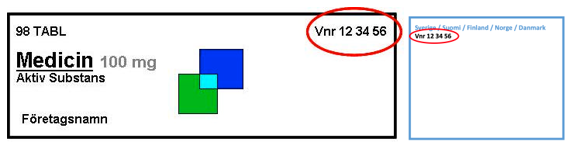
The six-digit number must be in three groups of two digits. In front of the number, it should read Vnr (without period).
Example: Vnr XX XX XX
For the Vnr to be clearly legible, it should be of a type size of at least eight points.
5.2 Allocation of the Vnr
The Vnr shall be placed:
- On a visible, and easily legible, place near the trade name
- Normally, on all sides where a complete identification of the package occurs.
The reason for this is that the Vnr is intended as an additional confirmation of the identity in the physical handling of the package. The Vnr must be easy to find and easy to read. An example of a good location and layout of a Vnr is in the top right corner on the front of the package.
For centrally authorised medicinal products, where labelling is not authorised nationally, it is common for the Vnr to be placed in a “blue-box”.
6 Pricing of Nordic Article Numbers
The cost for NNC is divided between the Nordic countries.
In Sweden, the handling of Vnrs is funded through the FASS-charge and therefore there is no direct cost associated with acquiring a Vnr. Marketing Authorisation Holders should regularly check their active Vnrs and withdraw all unnecessary numbers. It is the responsibility to withdraw a Vnr both in LiiV and in PICs system.
7 Use of article number series
The Nordic Article Number (Vnr) is an identification code for a specific article of medicine with marketing authorisation in the Nordic countries. The Vnr is a six-digit-code (000001-199999 and 370000-599999) given to all human and veterinary medicines. It enables a simple verification of packages at all stages in the drug supply chain from prescription to the patient.
The remaining six-digit-codes (200000-369999 and 600000-999999), which are not Nordic Article Numbers (000001-199999 and 370000-599999), are called National article numbers. They are used differently in the Nordic countries, for instance for consumer products (e.g. shampoo, etc.), medical devices (e.g. blood glucose test strips and colostomy bags), as well as for compounded drugs provided under special licence and extemporaneous preparations. This means that the same National article number is used for different articles in different countries.
In the table below all Swedish six-digit-codes are listed. It also shows what different number series are used for, and the owners of these series – January 2021, version 4.0.
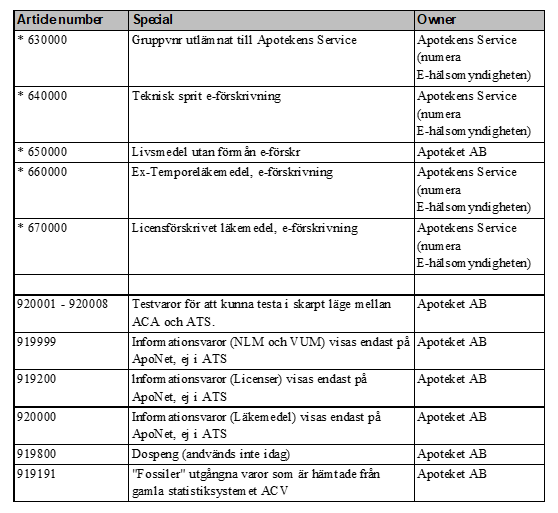
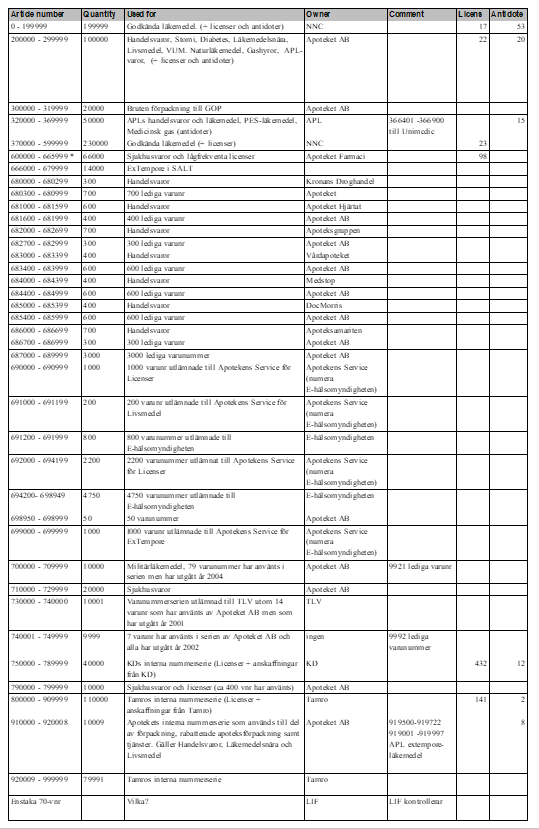
Table 1 List of all article numbers used in Sweden (”lediga varunr” not updated since 2012)
8 Läkemedelsbranschens nummernämnd, LNN
LNN is a forum in which the different stakeholders of the pharmaceutical and pharmacy industry can discuss the overall handling of article numbers. Currently, the forum is dormant, but will be activated when needed. LNN has the following members:
- Lif (Pharma companies)
- Medical Product Agency (Läkemedelsverket)
- Swedish eHealth Agency
- Swedish Pharmacy Association
- Wholesale distributors
- Dental and Pharmaceutical Benefits Agency (TLV)
- Swedish Association of Local Authorities and Regions (SKR)
- Extempore pharmacies
9 Contact information
The Vnr system is administered by the Nordic Number Centre (NNC)[2], located at Pharmaceutical Information Centre Ltd (PIC) (Lääketietokeskus Oy), Helsinki, Finland. vnr@vnr.fi
9.1 Sweden
Läkemedelsindustriföreningens Service AB, Lif
Box 17608
118 92 Stockholm
Telephone: 08-462 37 00
E-mail: fass@lif.se
Any questions about Vnrs and National article numbers in Sweden can be directed to the Swedish eHealth Agency: registrator@ehalsomyndigheten.se
Please read TLV’s document Handbok för företag vid ansökan om subvention och pris för läkemedel. The document is available on the website of TLV: Handbok för företag vid ansökan om subvention och pris för läkemedel (tlv.se)
Pharmacies approved by Medical Product Agency: http://www.lakemedelsverket.se/malgrupp/Apotek–handel/Apotek/-Tillstand-for-apotek/
Contact list to pharmacy chains when to inform about article information:
http://www.sverigesapoteksforening.se/apoteksbranchen/varuinformation/
10 Definitions
| FASS | The Swedish medicine compendium. |
| GS1 | GS1 is an international not-for-profit association with member organisations in over 100 countries. The GS1 standards, e.g. GTIN, are the most widely used supply chain standards in the world. www.gs1.se or www.gs1.com |
| GTIN | Global Trade Item Number – a global standard for article numbering administered by GS1. Consists of 13, maximum 14, digits on the format company´s prefix + serial number + check digit. Can be presented in a barcode for automatic reading. |
| Lif | Läkemedelsindustriföreningens Service AB, Lif, is the trade association for the research-based pharmaceutical industry in Sweden. www.lif.se |
| LiiV | Supplier information in the VARA register. The Swedish eHealth Agency is responsible for the development and administration of the product and article register LiiV and VARA |
| LV | Läkemedelsverket (MPA – Medical Product Agency) |
| MAH | Marketing Authorisation Holder |
| MPA | Medical Product Agency (LV – Läkemedelsverket) |
| Marketer | Company that represents the product (often the same as MAH) |
| National article number | A six-digit code (200000-369999 and 600000-999999) used for articles, which are not human or veterinary medicines. The same article number can be used for different articles in different Nordic countries. |
| NNC | The administration of the Nordic Article Number system is under responsibility of the Nordic Number Centre (NNC), located at Pharmaceutical Information Centre Ltd (PIC) (Lääketietokeskus Oy), Helsinki, Finland. vnr@vnr.fi |
| Nordic Article Number | Vnr – a six-digit identification code (000001-199999 and 370000-599999) for a specific article of medicine with marketing authorisation in the Nordic countries. A Vnr is given to all human and veterinary medicines. It enables a simple verification of articles at all distribution stages. |
| NPL id | An identification code for a product. It is generated and administered by MPA. |
| NPL pack id | An identification code for an article. It is generated and administered by MPA. |
| NTIN | National/Nordic Trade Item Number – Before February 2019 used instead of GTIN for pharmaceuticals in the Nordic countries. Same format as GTIN (13, maximum 14, digits) but with a fixed prefix and a Vnr instead of an article reference number. Format: prefix of Nordic Number Centre (704626) + Vnr + check digit
Since February 2019 no new NTINs are created. If an old NTIN is unique it can still be used, but it must follow the rules of a GTIN and thereby be changed according to the GTIN guidelines. |
| OTC | Pharmaceuticals available without a prescription. |
| PIC | Pharmaceutical Information Centre – http://www.laaketietokeskus.fi/en |
| Rx | Pharmaceuticals only available with a prescription |
| Sil | Svenska Informationstjänster för läkemedel |
| SKR | Swedish Association of Local Authorities and Regions (SALAR) – Sveriges Kommuner och Regioner (SKR) |
| TLV | Tandvårds- och läkemedelsförmånsverket – Dental and Pharmaceutical Benefits Agency, administers the prices in the reimbursement system. |
| Vnr | Nordic Article Number – a six-digit identification code (000001-199999 and 370000-599999) for a specific article of medicine with marketing authorisation in the Nordic countries. A Vnr is given to all human and veterinary medicines. It enables a simple verification of articles at all distribution stages. |
| VARA | The national product and article register for all pharmaceuticals, and consumer products within the reimbursement system. VARA is administered by the Swedish eHealth Agency www.ehalsomyndigheten.se |
[1] On behalf of the Nordic country organisations Dansk Lægemiddel Information A/S in Denmark, Pharmaceutical Information Centre Ltd in Finland, Lyfjastofnun in Iceland, Legemiddelindustriforeningen in Norway, and Läkemedelsindustriföreningens Service AB, Lif, in Sweden.
[2] On behalf of the Nordic country organisations Dansk Lægemiddel Information A/S in Denmark, Pharmaceutical Information Centre Ltd in Finland, Lyfjastofnun in Iceland, Legemiddelindustriforeningen in Norway, and Läkemedelsindustriföreningens Service AB, LIF, in Sweden.
