Multi packages
Country specific information: DENMARK
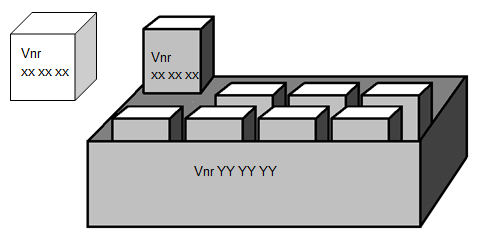
There are two kinds of multi packages.
The single packages of a multi package have market authorisation
A registered multi package can contain units with marketing authorisation as separate packages, e.g. 10×100 tablets.
The single packages within a multi package may have the same Vnr (Vnr XX XX XX) as the registered separate single packages on the market.
This does not apply to prescription-only medicinal products for pets in Denmark. See country specific information.
The Vnr of the multi package (Vnr YY YY YY) must differ, and may not appear on the single packages inside.
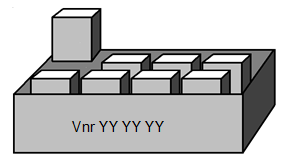
Only the multi package has a market authorisation
A registered multi package can contain units, without market authorisation. In this case the single packages must not have a Vnr, but the multi package must have a Vnr number (Vnr YY YY YY).
Country specific information: DENMARK